Early CDISC Trial Design drives study build. https://www.youtube.com/embed/TTqAsUn2aIY?rel=0
Category: Clinical Trials
Clinical Standardization Starts With Clinical Protocol
Start your End-to-End standardization from clinical protocol. https://youtube.com/watch?v=Fz6L2HuwzOI%3Frel%3D0
Agile Clinical Trials Planning and Design
Looking to improve the way your clinical trials are planned and designed? https://www.youtube.com/embed/3pDBoBeTOCs?rel=0
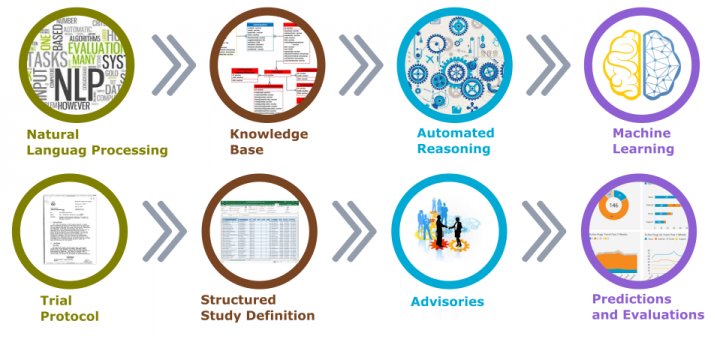
Artificial Intelligence in Clinical Trials Planning and Design
To effectively discuss the topic of Artificial Intelligence AI in Clinical Trials Planning and Design, we need to discus what […]
TransCelerate Common Protocol Template: Considerations for Sponsor Adoption
Assumption on my side is that if you are reading this article, then you probably are familiar with the TransCelerate […]
New Insights On Optimizing Protocol Design Practice To Maximize Development Performance
https://youtube.com/watch?v=1bIPs84LNgs%3Frel%3D0 Part 1 of 4: Ken Getz presentation, sponsored by intilaris LifeSciences titled ‘New Insights on Optimizing Protocol Design Practice […]
Meet ICH Quality Management Goals
ICH E6 (R2) provides meaningful additional guidance. In regards to improving study designs, we’d like to call your attention to […]
Effective use of CDISC Therapeutic Area Data Standards in the study setup
The Clinical Data Interchange Standards Consortium (CDISC) has, in recent years, released several Therapeutic Area User Guides (TAUGs) ranging from […]
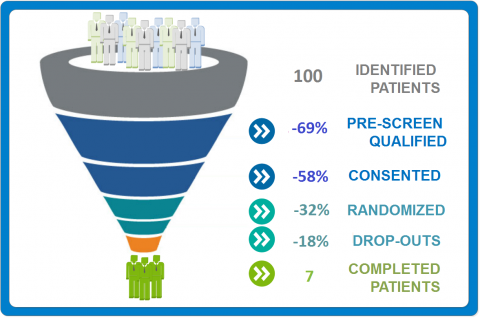
Improving Oncology Patient Enrollment
Success of a clinical study relies to big extent on enrolling patients. Nearly 25% of cancer trials fail to enroll […]
Standardized Trial Protocols: Service and Toolkit for generation of trial protocols based on Transcelerate template
The pharma industry is generally considered as an expensive enterprise that requires considerable investments into the R&D activities with virtually […]