Computer System Validation CSV Service
Computer System Validation CSV service is an independent service that supports CSV process used by regulatory agencies around the world to ensure and document that a computer-based system is performing as intended. In other words, that the data produced, and information derived from the system meet the predefined requirements in a consistent and reproducible manner.
CSV is required in the pharmaceutical, biologics, or medical devices industry, when developing or configuring a new computer system, or making a change in an already existing and validated system such as upgrades, patches, functional extensions, and others.
How intilaris reduces the customer burden by computer system validation CSV service?
Intilaris provides risk-based computer system validation CSV service according to GAMP category 4 and 5, i.e. configured and bespoke systems. We are capable to support your CSV through all phases end-to-end: from user requirements to system go-live. Our implementation managers are experienced in guiding customers through the validation process and ensure:
- Compliance
- Risk reduction
- Time saving
- Minimal validation cost
Throughout the implementation process, the implementation manager helps you to plan, configure, manage technology providers, test, and maintain your system to ensure that your system meets both your internal standards and regulatory requirements.
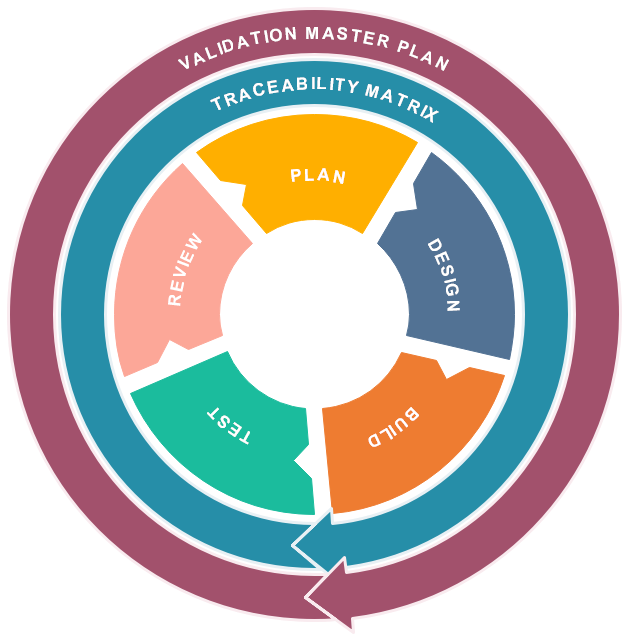
For best support of our customers along the V-model, our managed computer system validation CSV service is phased into 5 key phases:
- Plan
- Design
- Build
- Test
- Review
When required, intilaris can also support your technology evaluation process. Our approach to the evaluation process is such that it ensures the maximum re-usability of the items, concepts and deliverables used in the evaluation.
Computer system validation CSV service phases
Plan Phase
Your intilaris service delivery manager works with you to plan all the validation activities, including:
- Business Process Analysis
- System Inventory and Assessment
- Validation Master Plan
- Validation Risk Assessment
- Validation Traceability Matrix
- Configuration / Development Plans
- Test Plans
Furthermore, the service delivery manager will support you to consider all aspects of the business process, make sure that you have asked all the right questions and ensure that the validation methodology aligns with your SOPs and applicable regulations.
Design Phase
In the design phase, we help you to cover all aspects of the business process and capture all your requirements in the proper format for the project (e.g. traditional user requirements form, user stories, use cases, etc.). We make sure that the new system or the system under change is addressing each user requirements with appropriate functional, design or configuration requirements, depending on the software category. Within this phase, we deliver:
- User Requirement Specifications
- Functional Requirement Specifications
- Configuration Specification or Design Specification
Build Phase
Computer system validation service build phase supports our customers to effectively engage with the technology providers and maximize the use of their technology and software skills for the best fit with the business. This is a critical phase, in which our experts that talk both technology and business languages bridge the two organization for the smooth and streamlined system build. Any issue, not identified in this phase, has a potential to become a major risk to the project in the subsequent phases.
We use our decades of experience to mitigate all the risks involved in potential misunderstandings between the business and user needs and capabilities of developed / configured technology and provider resources. In the build phase we provide:
- Configuration of the system for GAMP 5 category 4 systems
- Guidance to technology providers and their teams for thorough and complete unit testing and integration testing for GAMP 5 category 5 systems
- Review of the technology documentation and alignment with specifications
Test Phase
This is the phase where all the good work executed in the earlier phase culminates in a successful qualification of the system’s correct functionality. Any gaps, or errors not caught in the earlier phases result in additional rework, delays, and additional costs. Therefore, it is of utmost importance that all the potentials issues have been discovered and dealt with through diligent focus and mechanisms in the preceding phases. The Installation qualification will confirm the correct installation and setup of the system with all its dependencies. The correct functionality of the system and the configuration is tested in the operational qualification and configuration qualification, respectively. Systems’ performance and user acceptance tests confirm that the system meets user needs and for their intended use as specified. In this phase, we would apply testing automation whenever possible and would follow use cases, SOPs, user defined scenarios, etc.
Major deliveries of this phase are:
- Installation Qualification (IQ) Tests and Results
- Operational Qualification (OQ) Tests and Results
- Performance Qualification (PQ) Tests and Results
- User Acceptance Tests (UAT) Tests and Results
Review Phase
When the system has been developed / configured and tested, we conduct the review phase in which we confirm by examination and provision of the objective evidence that the system meets the user needs and intended use. The outcome of this phase provides the following deliveries:
- Test Reports
- Validation Report
- System Release Documentation
- SOP Review (Update)
- Training Review
The Validation Report summarizes the testing results and provides confirmation that all acceptance criteria have been met and the software is ready for productive deployment. Any SOP updates and user training materials are also confirmed.
For an in-depth conversation on this topic, please reach out to us.
intilaris LifeSciences – LinkedIn

