TransCelerate Digital Data Flow Consulting
Recently launched initiative TransCelerate Digital Data Flow (DDF) aims to optimize and improve the clinical study start-up. Main intent is to engage with vendors, potential providers and others to define and create the optimal platform.
This topic is also very close to our heart as we have been focusing on the business process and change management part of this initiatives with our clients for years.
However, broad cross-functional change management and process alignments are essential for the success of this initiative in any pharma organization. Our clients benefit from our experiences in helping organizations transform and prepare themselves to benefit from this initiative.
For an in-depth conversation on this topic, please reach out to us.
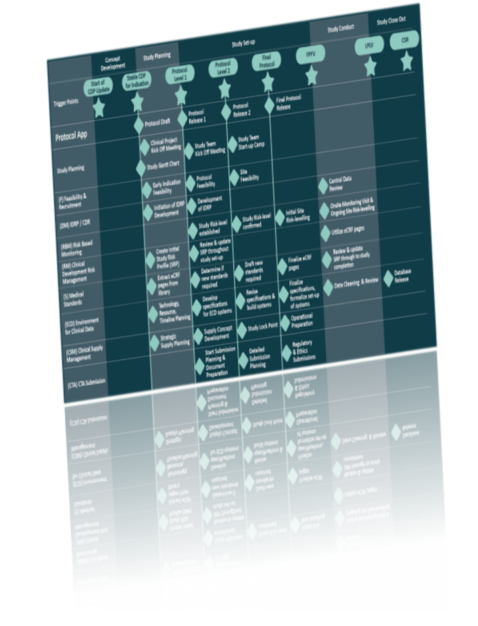
Current State of Industry Processes
The current state of the processes in the pharma industry typically involves disconnected study design services and assets, translation, transcription and re-entry of the planning and design information into many systems across internal sponsor processes and study collaborators like Contract Research Organizations. The result of this inherent inefficiency is manifested in systems configuration falling onto the critical path of the study start-up and creation of unnecessary delays and potential transcription errors.
In case of potential protocol amendments, the issues are multiplied as the systems would need to be re-configured resulting in a significant financial burden added to the clinical trial budget.
Future with TransCelerate Digital Data Flow
The solution proposed by TransCelerate Digital Data Flow would enable system interoperability between all processes in a clinical study, resulting in improved efficiency, data quality, transparency and reduction of cycle times. The solution will capture operational protocol elements and provide them in standardized formats to downstream processes and systems, enabling automated configuration and efficient provision of protocol information across the study ecosystem.
Organizational Change
The initiative is certainly aiming in the right direction to optimize and improve creation and distribution of the protocol information at the earliest point in time. For years now, we at intilaris have been working on structuring the protocol information and enabling downstream processes to benefit from it. We have been presenting at numerous conferences on this topic, creating awareness of these types of optimizations and the resulting benefits that we are seeing.
One thing that is yet lacking in the initiative is the people and process factor. Currently the initiative is strongly focused on the technology, but the organizational change is equally important. We have seen challenges that pharma organizations are facing when transitioning to structuring study planning and design processes and they need to be dealt with ahead of any technology introduction.
Cross-functional change management and process discussions are essential for the success of this initiative. In our past experiences we have engaged with many functions within the organization to align and facilitate a leaner process to execute DDF initiative, some of which are:
- Clinical Project
- Management
- Clinical Study Management
- Medical Writing
- Clinical Leaders
- Study Protocol Developers
- Target Product Profile Management
- Feasibility
- Drug Supply Management
- Standards Management
For an in-depth conversation on this topic, please reach out to us.
intilaris LifeSciences – LinkedIn

