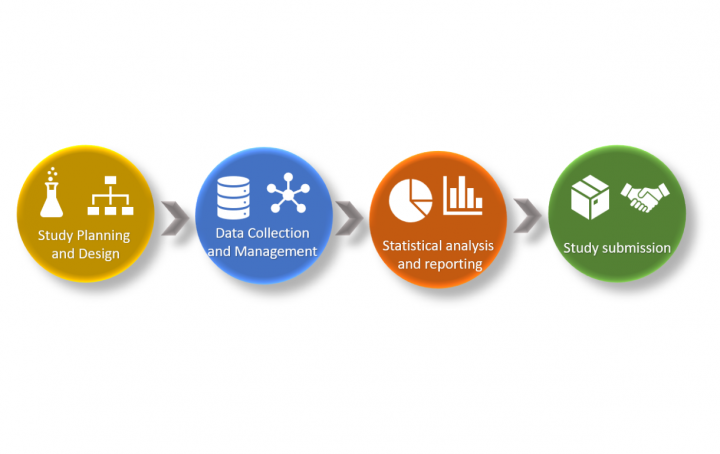
A seemingly simple question regarding the organization’s clinical data strategy reveals one of the still under-utilized opportunities for many pharma […]
Category: Clinical Trials
FDA TPP – Target Product Profile – Strategic Development Tool for Therapeutic Candidates
One of the tools that we have in Pharma R&D organizations at our disposal to manage strategic development for therapeutics […]
Digitalized Protocol Eligibility Criteria
Digitalized Protocol Eligibility Criteria enable both human readability but also immediate use in RWE tools in a digitalized form for […]
Keys to Clinical Development Excellence
Now, more than ever, It is becoming increasingly more evident that pharma companies in general and clinical development organizations in […]
Digital Transformation in Clinical Trials
It is becoming increasingly more evident that pharma companies clinical development organization must invest in digital transformation in clinical trials […]
Risk Management in Clinical Projects
Implementation methodology of the Risk Management in Clinical Projects requires coordination and integration of the information from several relevant sources. […]
Oracle LSH Consulting for Clinical Data Warehouse Platform
Clinical Data Warehouse – the most valuable asset in pharma company A clinical data warehouse is your company’s central clinical […]
MDR MetaData Repository: From an illusory to a true MDR
During the last CDISC EU Interconnect in Amsterdam 8-9 May 2019, I experienced a confusion among many attendees and even […]
E2E Clinical Data Standardization: Why we fail at the BEGINNING of it?
The Clinical Data Interchange Standards Consortium (CDISC) has, in recent years, released several Therapeutic Area User Guides (TAUGs) ranging from […]
Quality by Design in Practice: Structured Drug Development
Quality by Design in Practice https://youtube.com/watch?v=h72qVImKMPQ%3Frel%3D0 Quality by Design in Practice is a systematic approach to development that begins with […]