A seemingly simple question regarding the organization’s clinical data strategy reveals one of the still under-utilized opportunities for many pharma […]
Category: Clinical Standards
Oracle LSH Consulting for Clinical Data Warehouse Platform
Clinical Data Warehouse – the most valuable asset in pharma company A clinical data warehouse is your company’s central clinical […]
MDR MetaData Repository: From an illusory to a true MDR
During the last CDISC EU Interconnect in Amsterdam 8-9 May 2019, I experienced a confusion among many attendees and even […]
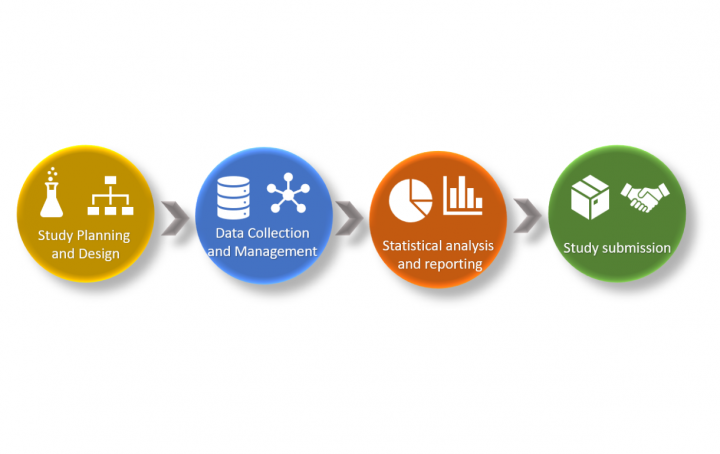
E2E Clinical Data Standardization: Why we fail at the BEGINNING of it?
The Clinical Data Interchange Standards Consortium (CDISC) has, in recent years, released several Therapeutic Area User Guides (TAUGs) ranging from […]
Effective use of CDISC Therapeutic Area Data Standards in the study setup
The Clinical Data Interchange Standards Consortium (CDISC) has, in recent years, released several Therapeutic Area User Guides (TAUGs) ranging from […]
Structured Protocol Framework reduces protocol standards obscurity
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Pellentesque ac urna in nunc blandit placerat. Aliquam hendrerit lacus euismod ipsum […]
MDR Implementation for Clinical Development
When one considers a Meta-Data Repository (MDR) implementation in the Pharma Industry today, one tends to focus immediately on the […]
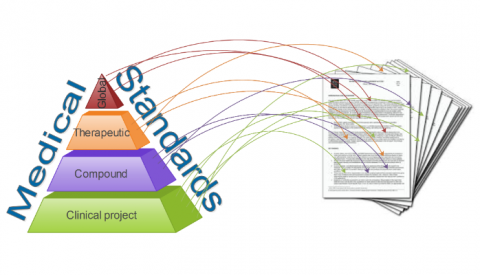
Is your organization making the most of Clinical Standards?
Many organizations find it hard to standardize their clinical data in the Clinical development Landscape. In reality, the standards repositories […]