The pharma industry is generally considered as an expensive enterprise that requires considerable investments into the R&D activities with virtually […]
Blog
Prevent Clinical Study Protocol Amendments
Protocols have become more demanding and complex and as a result more amendments arise. In fact, ‘the majority of clinical […]
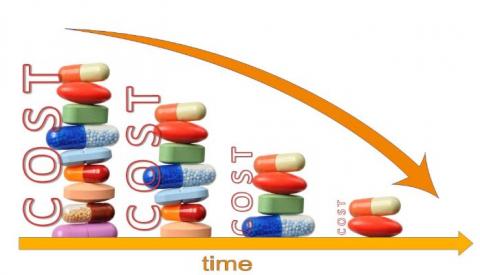
Clinical Protocols : Improve your designs and reduce costs
The studies executed by the Tufts Center for the Study of Drug Development (Tufts CSDD), clearly show the dependency between […]
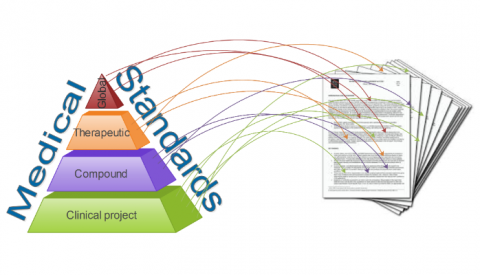
Structured Protocol Framework reduces protocol standards obscurity
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Pellentesque ac urna in nunc blandit placerat. Aliquam hendrerit lacus euismod ipsum […]
MDR Implementation for Clinical Development
When one considers a Meta-Data Repository (MDR) implementation in the Pharma Industry today, one tends to focus immediately on the […]
Is your organization making the most of Clinical Standards?
Many organizations find it hard to standardize their clinical data in the Clinical development Landscape. In reality, the standards repositories […]