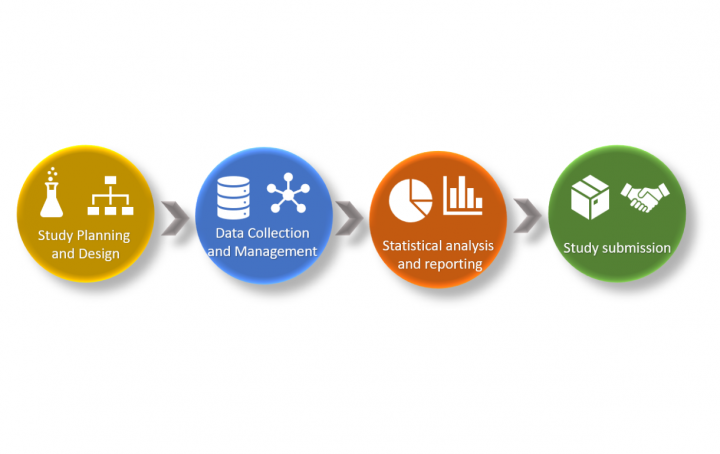
During the last CDISC EU Interconnect in Amsterdam 8-9 May 2019, I experienced a confusion among many attendees and even […]
Tag: cdisc
E2E Clinical Data Standardization: Why we fail at the BEGINNING of it?
The Clinical Data Interchange Standards Consortium (CDISC) has, in recent years, released several Therapeutic Area User Guides (TAUGs) ranging from […]
From Clinical Protocol to CDISC Trial Design
Early CDISC Trial Design drives study build. https://www.youtube.com/embed/TTqAsUn2aIY?rel=0
Effective use of CDISC Therapeutic Area Data Standards in the study setup
The Clinical Data Interchange Standards Consortium (CDISC) has, in recent years, released several Therapeutic Area User Guides (TAUGs) ranging from […]
Is your organization making the most of Clinical Standards?
Many organizations find it hard to standardize their clinical data in the Clinical development Landscape. In reality, the standards repositories […]