Quality by Design in Practice https://youtube.com/watch?v=h72qVImKMPQ%3Frel%3D0 Quality by Design in Practice is a systematic approach to development that begins with […]
Category: Clinical Study Protocols
From Clinical Protocol to CDISC Trial Design
Early CDISC Trial Design drives study build. https://www.youtube.com/embed/TTqAsUn2aIY?rel=0
Clinical Standardization Starts With Clinical Protocol
Start your End-to-End standardization from clinical protocol. https://youtube.com/watch?v=Fz6L2HuwzOI%3Frel%3D0
Agile Clinical Trials Planning and Design
Looking to improve the way your clinical trials are planned and designed? https://www.youtube.com/embed/3pDBoBeTOCs?rel=0
TransCelerate Common Protocol Template: Considerations for Sponsor Adoption
Assumption on my side is that if you are reading this article, then you probably are familiar with the TransCelerate […]
New Insights On Optimizing Protocol Design Practice To Maximize Development Performance
https://youtube.com/watch?v=1bIPs84LNgs%3Frel%3D0 Part 1 of 4: Ken Getz presentation, sponsored by intilaris LifeSciences titled ‘New Insights on Optimizing Protocol Design Practice […]
Meet ICH Quality Management Goals
ICH E6 (R2) provides meaningful additional guidance. In regards to improving study designs, we’d like to call your attention to […]
Prevent Clinical Study Protocol Amendments
Protocols have become more demanding and complex and as a result more amendments arise. In fact, ‘the majority of clinical […]
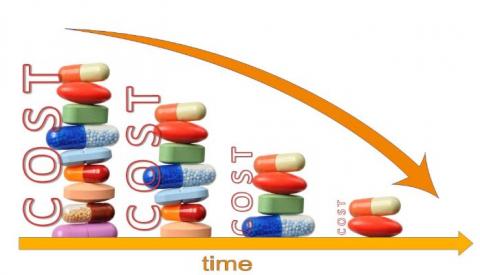
Clinical Protocols : Improve your designs and reduce costs
The studies executed by the Tufts Center for the Study of Drug Development (Tufts CSDD), clearly show the dependency between […]
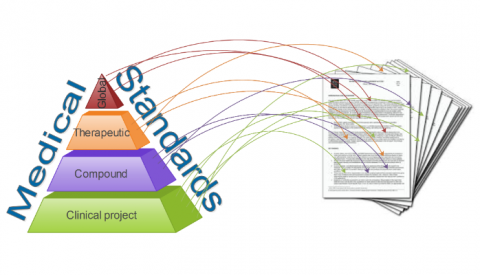
Structured Protocol Framework reduces protocol standards obscurity
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Pellentesque ac urna in nunc blandit placerat. Aliquam hendrerit lacus euismod ipsum […]