An effective product portfolio strategy for any R&D organization must include strategies to optimize Target Product Profile – TPP of their development candidates. The optimized TPPs are used to manage the R&D portfolio by evaluating, prioritizing and selecting development projects.
Why you must Optimize Target Product Profile
According to the FDA Guidance Document, Target Product Profile is a useful Strategic Development Process Tool to facilitate communication regarding a particular drug development program [1]. The importance of the TPP in the focus and facilitation of the FDA approval is no doubt quite significant as it contributes to the reduction of the review time [2]. However, even though the facilitation of the FDA review through a TPP is advantageous it still remains underutilized [3]. This under-utilization is perhaps due to the lack of the vision on how one can optimize target product profile and use it as the main driver for the entire product development. Later on, we will outline how one can use TPP to assess the value of development candidate and how that value can be optimized through a holistic look at the TPP.
Target Product Profile contribution to the specification and management of the overall commercial strategy is equally important as the commercial success is typically the final development goal. The lack of commercial success has been seen in numerous examples of bio-pharmaceutical products that have been developed, approved and launched. TPP enables you to start with end in mind and to define, align and manage your product development to the agreed goal with the right messaging, post-launch data and services differentiators. Bain&Company research has indicated that that nearly 50% of launches over the past eight years from 2017, have under-performed analyst expectations, and more than 25% have failed to reach even 50% of external revenue forecasts. In other words, a proper definition and assessment to optimize Target Product Profile enables R&D organizations to better adopt the ‘Commercially Driven’ bio-pharmaceutical product development.
Systemic approach to Optimize Target Product Profile
The FDA Guidance Document [1] includes the TPP template that can be adopted and utilized for definition of the TPP. Indeed, many companies are adopting different variations of that template to capture the main profile characteristics and differentiators (value indicators) of the new product.
However, what is largely missing is the systemic view of the profile value indicators, their interconnections and dependencies and ability to rate and compare within the TPPs life-cycle and with competitors’ TPPs. While defining, evaluating and optimizing the TPP, one must not ignore the relationships between the profile value indicators as changing and affecting one of the indicators, could produce a totally different outcome due to the complex nature of that system.
Therefore, one must approach the TPP from the systems thinking (holistic) viewpoint. Such approach will be able to aid the TPP optimization in such a way to precisely point out in which areas an improvement is needed in order to affect the desired product properties.
For example, to optimize Target Product Profile with respect to ‘Patient Recruitment’ requires adjustments to other closely related product properties and their value indicators.
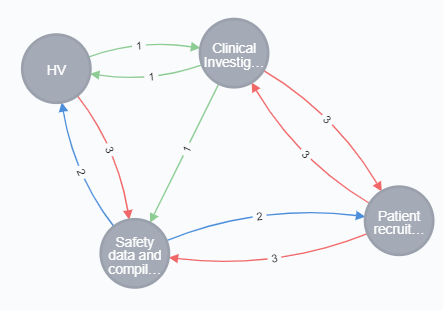
How to get started to Optimize Target Product Profile
One of the main challenges in adopting and utilizing a holistic approach to evaluate and optimize target product profile is in:
- Lack of resources familiar with System’s Thinking concepts to apply it to TPP
- Complexity of the R&D system makes the modeling and simplification a cross-collaborative task and effort with a right team mindset, which is not readily available
- Lack of a working model, which organizations can apply to this problem
For any internal R&D team, it is quite a challenge to transition these challenges. Therefore, intilaris, has been investing in reducing the effect of these obstacles, by developing a TPP model that can be applied by any internal R&D team familiar with the product under development. Such a collaborative approach ensures adequate and consensual assessment of the product value and alignment of all in the team on the product strategy.
At intilaris, we have been working extensively with great customers and collaborators to understand and model the complexity of the drug development R&D into a Systems Thinking model that can be applied by the R&D teams. We have defined a working TPP model consisting of 27 variables indicating the value of the future product in all aspects of the R&D. Our TPP model, simplifies the complexity of the drug development R&D, yet can be effectively used to predict the outcomes of changes to the product under development with all of its dependencies and relationships between variables (value indicators). The model can be applied by your internal R&D teams and can be used to assess, monitor, and optimize target product profile and ensure commercial success of your future bio-pharmaceutical product. The application of Systems Thinking approach to TPP is executed in 3 steps:

Please reach out to us for an independent and expert consultation on these topics.
References
[1] Guidance for Industry and Review Staff Target Product Profile — A Strategic Development Process Tool, https://www.fda.gov/media/72566/download (Accessed 2-Jul-2019).
[2] Breder, C.D., Du, W., Tyndall, A. What’s the Regulatory Value of a Target product Profile? Trends in Biotechnology 2017; 35, 576 – 579.
[3] Tyndall A. et al. Regulatory watch: the target product profile as a tool for regulatory communication: advantageous but underused. Nat. Rev. Drug Discov. 2017; 16: 156
–


