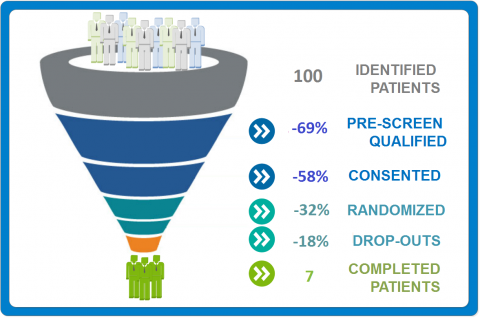
Success of a clinical study relies to big extent on enrolling patients. Nearly 25% of cancer trials fail to enroll the required number of patients and only 3% of adult cancer patients in the U.S. participate in clinical trials. The remaining 97% of cancer patients are not reached to participate in clinical trials.
Even when the sponsor succeeds with their patient enrollment targets, there is another obstacle, that is patient retention; the ability to keep patients from start to finish. According to Forte Research Systems, 85% of clinical trials fail to retain enough patients.
Poor enrollment and retention rates lead to increased trial costs, delays and compromised data quality. With this much at stake, sponsors should reconsider the level of trial planning and targeted patient enrollment. On protocol level, this could be achieved by:
- Minimizing burdens during protocol design
- Early and comprehensive feasibility assessments
- Refined and yet effective inclusion/exclusion criteria
intilaris has extensive experience in designing oncology studies. intilaris Structured Protocol Framework service combines resources and tools that help increase the success of your oncology study through:
Feasibility through timely generated draft trial concepts
intilaris concept of structured study definition facilitates the important assessment of the trial feasibility through timely generated draft trial concepts. The sponsors are then able to engage early with investigators, participating sites and patient communities. This early feedback from different stakeholders brings the crucial insight into the study planning phases leading to feasible study designs and protocols. It also facilitates definition and effective refinement of the inclusion and exclusion criteria, which are the key to higher recruitment figures.
Structured study definition also helps to determine the list of trial countries. It allows, via the refined target population, identification of potential trial sites. Other sponsors might be already active there and patients are enrolled in competitive trials. The feasibility assessment should consider this as well. A structured study definition can drive an effective feasibility and find the right patients in fewer countries and sites with a larger number of patients.
Structured and standardized trial designs, effective protocols
The identification of critical trial design elements enables clinical study teams to address the protocol design complexity and raise concerns upfront regarding patient procedures. This helps to identify the right patient population, the right sites and to reduce complex and burdensome procedures. Structured and standardized study designs require fewer amendments, assure higher accrual rates of the study participants and help to keep the targeted timelines. Furthermore, patients are more likely to participate and stay in a less complex study.
*Source: Forte Research Systems