Digital clinical trial protocol in the context of this article represents a digital asset of the sponsor company, that contains all executable information required to conduct the clinical trial both internally and externally. As a digital asset, the protocol can be made available in many different forms depending on the use case in which the information is consumed. Furthermore, the digital protocol development process should not follow the traditional protocol development process but should rather employ Agile Methodologies which would enable early value derivation and process optimizations across clinical operations.
Many sponsors have recognized the need to apply digital technology and analytics capabilities in the protocol design process and are developing digital strategies to address that need. However, many of these initiatives are focused on artificial intelligence (AI) applications to analyze big data pools of clinical facts to support more efficient protocol design and patient recruitment. As helpful as these technologies can be, we are still treading the same path, albeit with better information, of creating documents to drive internal processes. Such an approach will not yield significant improvements in clinical operations except the potential of better addressing the patient recruitment if data on which AI technology is well structured which is often not the case.
Operationally, a lean operational model with a well-structured protocol digital asset and appropriate KPIs in place, will win in the productivity race over ‘throwing in’ AI technology to try to make a better sense of the unstructured data.
At intilaris, we have been supporting our clients in transitioning their protocol development processes and clinical operations integration into digital space. The greatest challenge in this transformation, as we have experienced it, is a successful transformation of the entire organization with tools, resources, processes to the ‘digital’ way of working.
Traditional Document Driven Protocol Development Process
Creating a clinical trial protocol is typically the activity that represents the start of the clinical trial. In it’s most recognizable form as a document, the protocol describes the trial objectives, rationale, and the need for the proposed research, target patient population, scheduled patient visits, activities, and outcomes. To create a protocol document is a complex endeavor that is ripe with opportunities to streamline.
Descriptions of the target patient populations are formalized through Inclusion-Exclusion (IE) criteria, which is usually non-standardized. As a result, we have IE criteria that contain verbiage statements of the kind that depends on the team or a person that formulated it.
Patient visit schedule and activities and procedures that are scheduled reflect the needs of the trial design but with no formalized relationships to the endpoints and/or trial objectives. This unstructured way of designing the trial, therefore, comes with the inherent risks of data points misalignment in the trial pooling and meta-analysis. It is, therefore, not surprising that we experience a significant increase in protocol design complexity [1].
All clinical development operation processes would typically start after the approval of the protocol document and would depend on the correct interpretation of the protocol content. As you can imagine such a traditional process does not offer opportunities to front-load operational activities and check protocol assumptions regarding the trial design.
Digital Clinical Trial Protocol Twin
R&D companies recognize the immense potential of simplifying and streamlining the protocol development process. We see that simplification and streamlining implemented through two core components:
- Executable study concept model
- Lean and integrated development process
The model for the executable study concept is the essential step toward digital clinical trial protocol and is required to be simple enough, yet complete enough to enable full integration of the clinical operations processes. This model, can be instantiated in a digital form and can serve as a digital asset and a single source of design truth for the entire organization involved in the clinical trial: planning, design, conduct, data management, analysis and reporting. In other words, it becomes the digital twin of the clinical trial protocol.
Once the trial design data is made available digitally, all sorts of optimizations and innovations become available. For example, the digital protocol can automate study build in the data collection, management and analysis systems through appropriate digital data flows and standards. The patient recruitment issues [2] can be effectively addressed through early verification of the target patient profiles using real-world data (RWD) rather than the traditional IE criteria.
Digital protocol asset offers visibility and transparency in the trial design, however despite that amendments to the design might arise as we learn more about the design specificity and need to make changes. Traditional protocol amendments have a significant impact on the trial performance and cost [3]. Once the amendments are made and new learnings about trial and indications are obtained, they can be linked to the digital protocol elements, so that the future protocols avoid similar issues. This way, the protocol design system would be able to learn from the mistakes and be able to advise future designs of the potential amendments issues.
These are just some examples of how digital protocol assets can improve clinical operations in general and contribute to lowering the cost of development and thereby the resulting medicine.
Lean and Agile Clinical Operations
The lean and integrated development process is the other core component of the simplified and streamlined protocol development process. It starts with an initial digital protocol and transforms and enriches its information content through development milestones. At each milestone, a set of digital protocol elements reaches its stability level and can be exchanged with downstream processes to front-load their activities.
Such a lean clinical operations process provides the operational organization with agility to address the productivity and success rates of their clinical trials once they go into execution. Properly aligned with downstream processes and applying agile methods the organizations can instantly share the digital twin of their clinical protocol across the silos and provide end-to-end transparency in the entire value chain and all stakeholders.
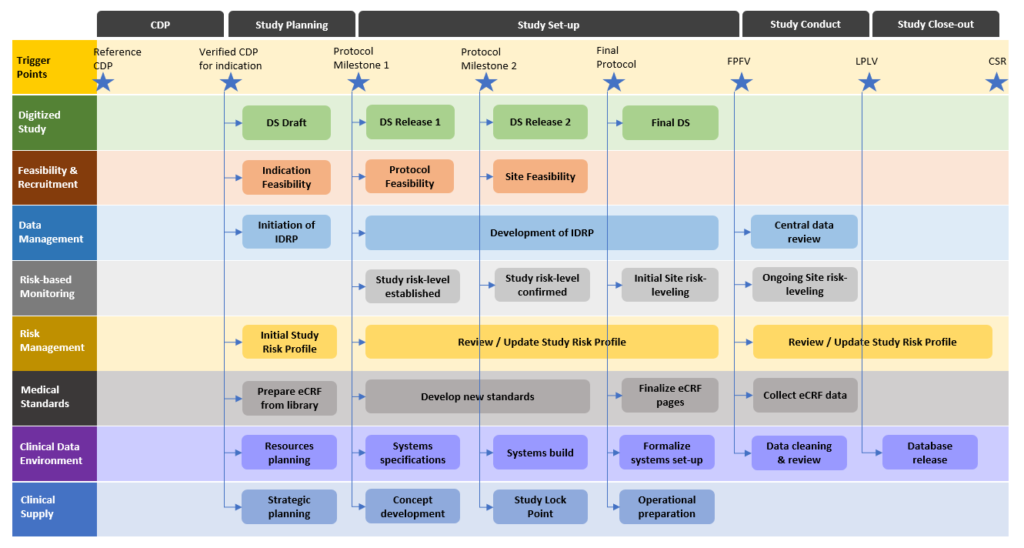
Due to different operational models across the industry, it is of utmost importance that all stakeholders are properly aligned and that the clinical trial protocol digital twin is carefully designed to ensure the full usability across the organization. That might require starting to discuss topics that the organization did not address previously in such an early stage. However, by doing so, the entire clinical operations value chain will operate a lot more effectively and giving more and more value at each iteration.
The lean and agile clinical operation process, combined with clinical trial protocol digital twin, is the future-proof way forward for not only big pharma organizations, but equally so for smaller pharma and biotech companies. The difference would be in the stakeholders’ value chain and internal versus outsourced services integration, but the overall principle remains to a large extent the same.
intilaris has been active over past years in bringing digital clinical trial protocol capabilities to research organizations and contributing to the industry in transforming its digital data flows from end-to-end.
Our support team is ready to help you in your digital transformation of the clinical operation processes using digital clinical trial protocol.
[1] Getz, K. A. and Campo, R. A. (2018) ‘New Benchmarks Characterizing Growth in Protocol Design Complexity’, Therapeutic Innovation & Regulatory Science, 52(1), pp. 22–28.
[2] Thoma, A., Farrokhyar, F., McKnight, L., & Bhandari, M. (2010). Practical tips for surgical research: how to optimize patient recruitment. Canadian journal of surgery. Journal canadien de chirurgie, 53(3), 205–210.
[3] Getz, K. A., Stergiopoulos, S., Short, M., Surgeon, L., Krauss, R., Pretorius, S., Desmond, J., & Dunn, D. (2016). The Impact of Protocol Amendments on Clinical Trial Performance and Cost. Therapeutic innovation & regulatory science, 50(4), 436–441.


